Étude ENGOT-en11
Fiche descriptive de l'étude
Étude Endomètre
Étude ENGOT-en11
Titre de l'étude
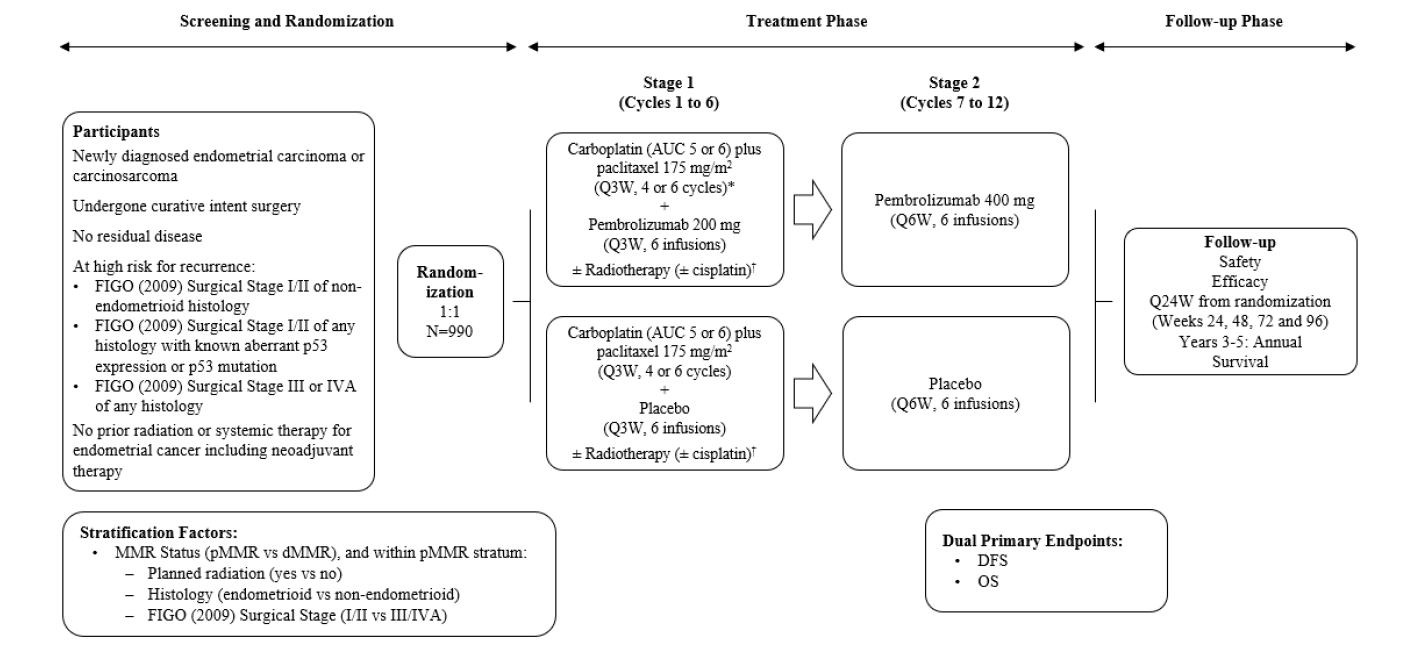
ENGOT-en11 : A Phase 3, Randomized, Double‐Blind Study of Pembrolizumab versus Placebo in Combination With Adjuvant Chemotherapy With or Without Radiotherapy for the Treatment of Newly Diagnosed High‐Risk Endometrial Cancer After Surgery With Curative Intent
Statut
Recrutement terminé / Suivi
Schéma de l'étude
Promoteur
MSD
But
Objectif principal :
Disease Free survival as assessed radiographically by the investigator or by pathologic confirmation and Overall Survival
Objectif secondaire :
Disease Free survival as assessed radiographically by blinded independent central review (BICR) or by pathologic confirmation and Disease Free Survival at 2 years assessed by investigator and assessed by BICR. Overall Survival at 3 years. Adverse events.
Phase
Phase III
Type de patiente
Patients with newly diagnosed, high-risk endometrial cancer (no prior radiation or systemic therapy for EC including neoadjuvant therapy).
Nombre de patientes recrutées
- 1090 patientes à l'international dans 220 centres
- 48 patientes en France dans 12 centres
Critères principaux d’évaluation
For DFS : The time from randomization to local or distant recurrence of endrometrial cancer, or death due to any cause, whichever occurs first. For OS : the time from randomization to death due to any cause.